Quaternary Structure of Human Hemoglobin Is Best Described as
A B -- C ΔG -1. -Many proteins are composed of multiple polypeptide chains called subunits individual peptide chains - and these proteins display quaternary structure.

Hemoglobin Structure Confirmation Binding And Transportation Of Oxygen The Science Notes
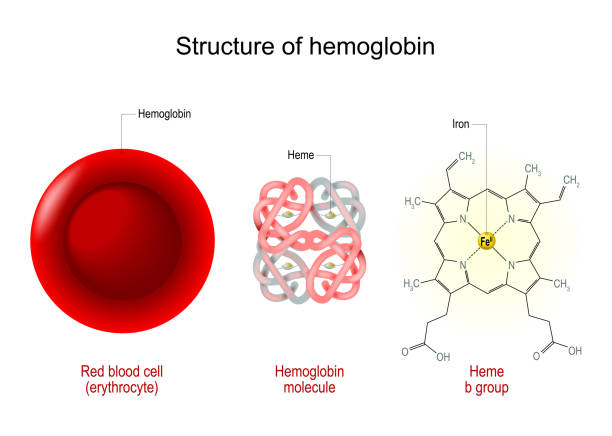
Hemoglobin commonly symbolized by Hb sometimes Hgb is an iron-containing metalloprotein found mainly in the blood of vertebrates within their red blood cells as well as in the tissues of certain invertebrates.

. Human hemoglobin HbA is one of the prototypal systems used to investigate structurefunction relationships in proteins. Which of the following statements best describes the shape and location of the heme groups. Which of the following statements best describes the shape and location of the heme.
Here we demonstrate that the quaternary structure of tetrameric human normal adult carbonmonoxy-hemoglobin can readily be determined in solution at near-physiological conditions of pH ionic strength and temperature by NMR measurement of 15N-1H residual dipolar couplings in weakly oriented samples. Hence two subunits of one type and two subunits of another type together constitute the human haemoglobin Hb. A tertiary protein will commonly contain a single polypeptide chain with one or more secondary structures.
Double-click on one of the gray heme group atoms in the model above and zoom in to examine it. Note the larger central water cavity in the T structure. There are 141 and 146 amino acids in the α and β chains of hemoglobin respectively.
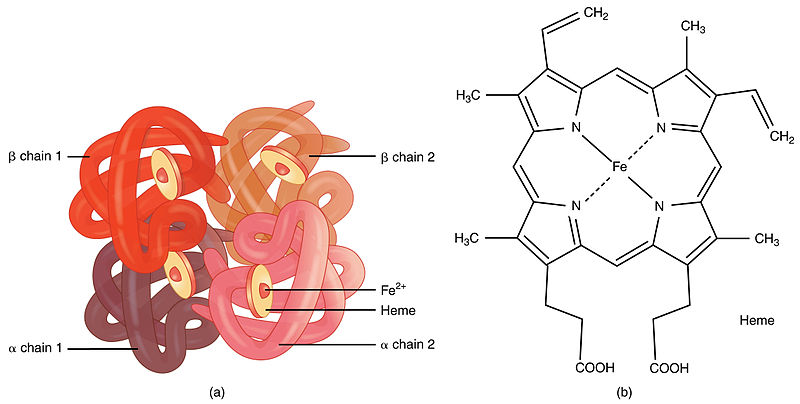
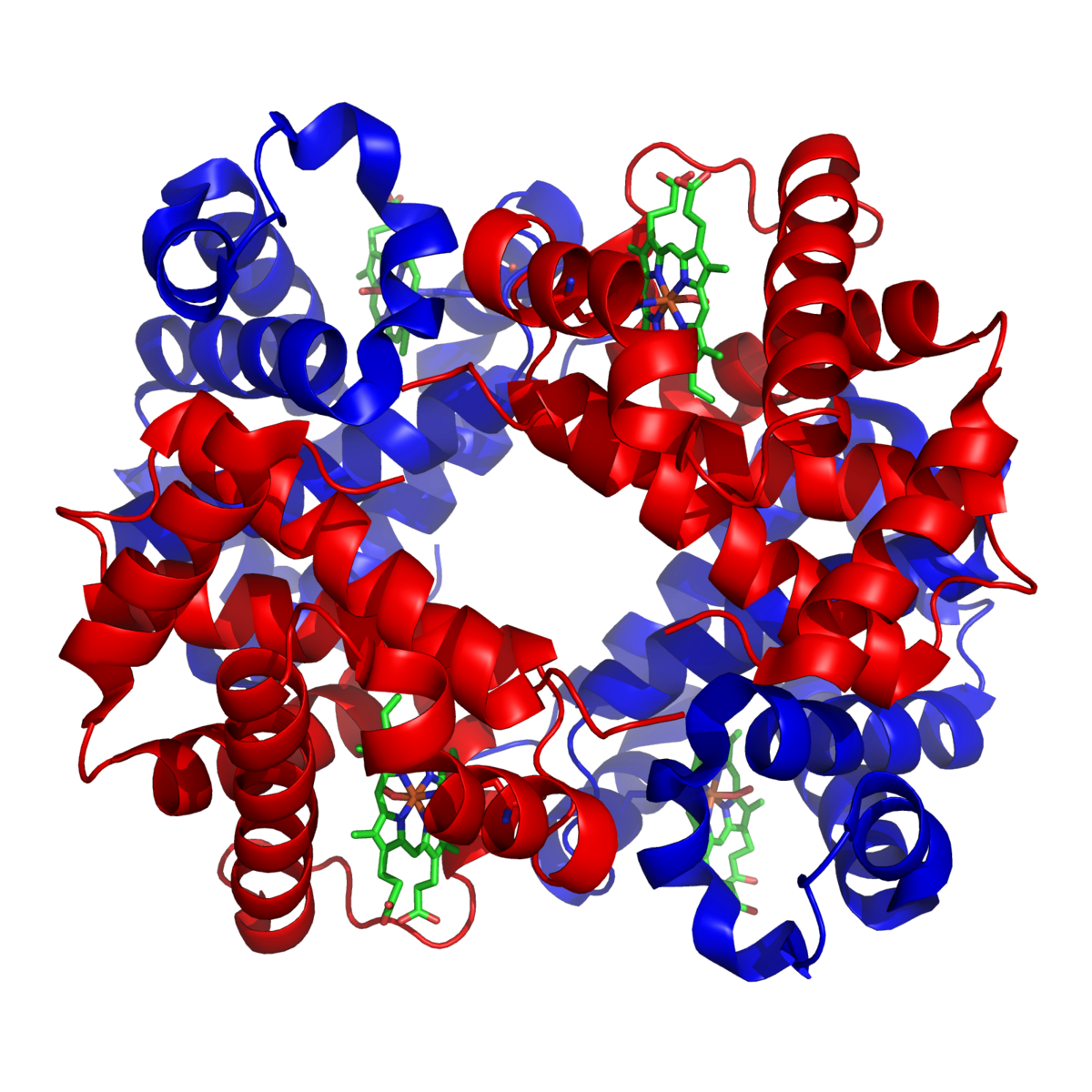
In human hemoglobin the fit between the polypeptide chain is critical because the gap between two of the polypeptide chains in the hemoglobin molecule becomes narrower when oxygen molecules become attached to the ferrous atoms. Quaternary Structure Transitions of Human Hemoglobin. It consists of two pairs of different proteins designated the α and β chains.
Hemoglobin has a quaternary structure characteristic of many globular subunit proteins. -Can be as simple as two identical polypeptide. -Highest level of some proteins.
An Atomic-Level View of the Functional Intermediate States. Hemoglobin is made up of four monomeric subunits each of which is known as a polypeptide and about the size of many normal individual proteins. Quaternary Structure Regulates Hemin Dissociation from Human Hemoglobin.
Individually each alpha helix is a secondary polypeptide structure made of amino acid chains. Human hemoglobin HbA is one of the prototypal systems used to investigate structure-function relationships in proteins. The quaternary structure of hemoglobin includes the assembly of two α and two β subunits each containing a heme group that is essential for oxygen binding.
Chapter 2 - Quaternary Structures. The quaternary structure of hemoglobin includes the assembly of two α and two β subunits each containing a heme group that is essential for oxygen binding. Quaternary structure involves two ormore strings of amino acids coming together.
It consists of two pairs of different proteins designated the α and β chains. Here we demonstrate that the quaternary structure of tetrameric human normal adult carbonmonoxy-hemoglobin can readily be determined in solution at near-physiological conditions of pH ionic strength and temperature by NMR measurement of 15 N-1 H residual dipolar couplings in weakly oriented samples. A series of reactions have the following ΔG.
Thus hemoglobin binds four O 2 molecules. Each one has atertiary structure but together they. Indeed HbA has been used to develop the basic concepts of protein allostery although the atomic-level mechanism.
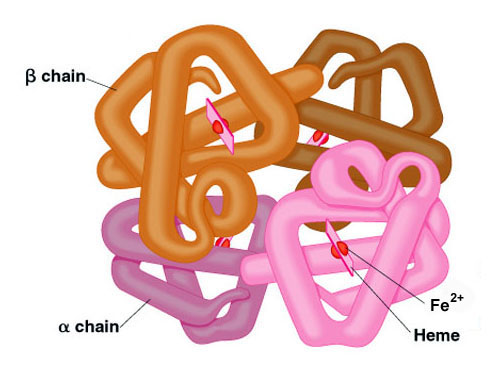
Haemoglobin consists of 4 subunits. Crystal structure of hemoglobin. The quaternary structure of a hemoglobin molecule includes four tertiary structure protein chains which are all alpha helices.
Hemoglobin is structurally similar to myoglobin used to store oxygen in muscles. -Based on arrangement of peptide chains. Hemoglobin has a quaternary structure.
The structure is found to be a dynamic. Thus hemoglobin binds four O 2 molecules. A Overall quaternary structure of Hb with the two α chains and β chains colored grey and tan respectively.
B Structure of oxygenated R state Hb magenta superimposed on the structure of deoxygenated T state Hb blue. Hemin dissociation rates were also measured for native isolated α and β chains and for recombinant hemoglobin tetramers. The correct option is D heterogenous tetramer.
Human hemoglobin Hb is a benchmark protein of structural biology that shaped our view of allosterism over 60 years ago with the introduction of the MWC model based on Perutz structures of the. As in myoglobin each subunit is linked covalently to a molecule of heme. Hemoglobin has a quaternary structure.
Up to 10 cash back Hemoglobin is a tetramer that possesses a quaternary structure containing multiple folded polypeptide structures tertiary structures. There are 141 and 146 amino acids in the α and β chains of hemoglobin respectively. Hemoglobin a globular protein that transports oxygen in blood consists of four polypeptide chains.
Rate constants for hemin dissociation from the α and β subunits of native and recombinant human hemoglobins were measured as a function of protein concentration at pH 70 37 C using H64YV68F apomyoglobin as a hemin acceptor reagent. C D -- E ΔG 3. Each of these subunits has its.
The arrangement of the subunitswhich is known as the quaternary structurediffers in the oxy- and deoxyhemoglobin. Rate constants for hemin dissociation from the α and β subunits of native and recombinant human hemoglobins were measured as a function of protein concentration at pH 70 37 C using H64YV68F apomyoglobin as a hemin acceptor reagent. Polypeptide chains or subunits the structural level is referred to as a quaternary structure.
As in myoglobin each subunit is linked covalently to a molecule of heme. Double-click on one of the gray heme group atoms in the model above and zoom in to examine it. Indeed HbA has been used to develop the basic concepts of protein allostery although the atomic-level mechanism underlying the HbA functionality is still highly debated.
Two of these are identical to each other. For examplehemoglobin is made up of four different proteins. Complex II which is also part of the TCA cycle is embedded in the inner mitochondrial membrane but this complex does not span the membrane therefore H are not pumped when electrons are passed through this complex.

Hemoglobin An Overview Sciencedirect Topics

Hemoglobin Gambar Foto Vektor Stok Shutterstock

Hemoglobin Has Quaternary Structure A True B False 2 Individual Alpha Helices Found In Brainly Com

Hemoglobin An Overview Sciencedirect Topics
Protein Structure Cell Crystal Structure Hemoglobin Png 500x500px Structure Biology Body Jewelry Cell Chemical Reaction Download
Hemoglobin Facts Structure Summary Synthesis Function

Structure And Function Of Haemoglobin Deranged Physiology
Hemoglobin And Myoglobin The Medical Biochemistry Page
1 411 Hemoglobin Molecule Stock Photos Pictures Royalty Free Images Istock

Are You Getting Enough Protein Baamboozle

Direct Observation Of Conformational Population Shifts In Crystalline Human Hemoglobin Journal Of Biological Chemistry

Hemoglobin Structure What Are The 4 Structures Of Protein Video Lesson Transcript Study Com

Quaternary Structure An Overview Sciencedirect Topics

Structures And Oxygen Affinities Of Crystalline Human Hemoglobin C B6 Glu Lys In The R And R2 Quaternary Structures Journal Of Biological Chemistry
Serum Darah Wikipedia Bahasa Indonesia Ensiklopedia Bebas

Structure And Function Of Haemoglobin Deranged Physiology

The Quaternary Structure Of Hemoglobin And Its Oxygen Carrier Heme Download Scientific Diagram
What Is The Structure Of Hemoglobin And How Is Oxygen Bound To It Quora

Comments
Post a Comment